

………first we need to know whether the system is actually two-‐phase.
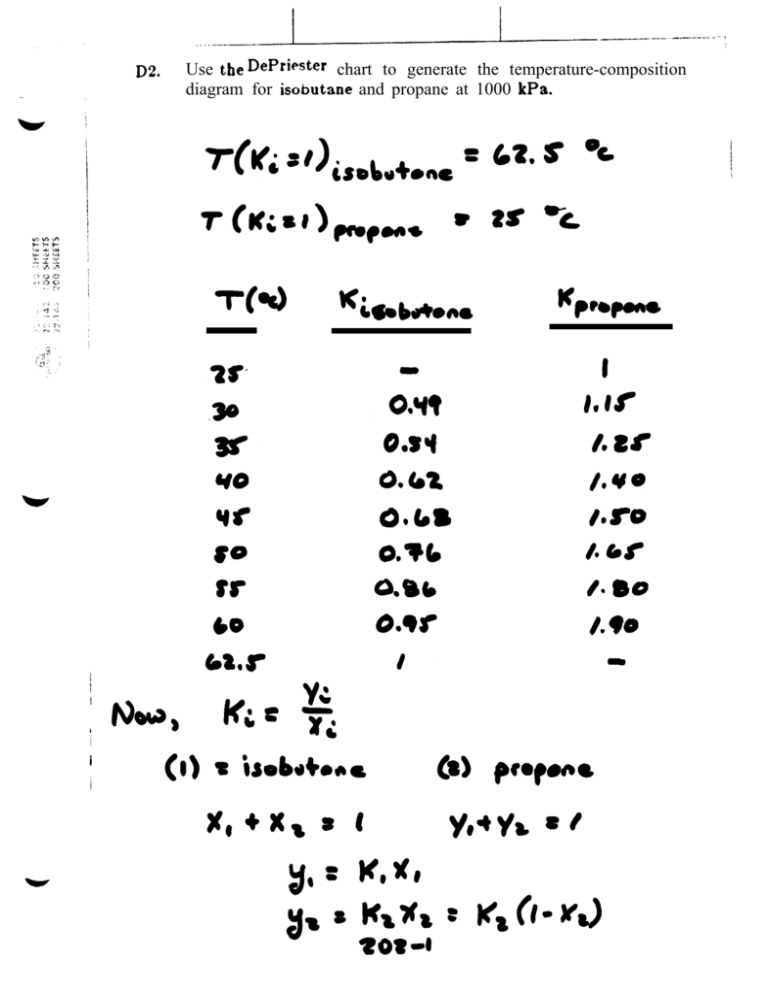
Solution is by trial and error.! Guess V until the summation term equal to 1.! But………! Ki are tabulated in a chart called the DePriester chart.īUBL P: Calculate composition of vapor !Īs derived for VLE system, zi K i yi = 1 + V (K i − 1) K-‐value Using DePriester Chart For light hydrocarbon mixture (commonly found in industry), Ki is essentially function of T and P only. Pi sat Ki = P If Modified Raoult’s Law is valid, Φˆi yi P = γ i xi f i yi P = γ i xi Pi sat Modified Raoult’s Law φˆi = 1 For ideal-‐gas mixture in equilibrium with non-‐ideal liquid solu6on Raoult’s Law For ideal gas vapor mixture in equilibrium with ideal liquid solu6on equation becomesĪnd also for pure species in equlibrium and ideal gas vapor, f i = f i l = f i v = P = Pi sat we get, Where, φˆi fugacity coefficient species i in gas mixtureĪctivity coefficient of species i in liquid solution Consider a multicomponent system in a VLE! condition, the fugacity (to be defined in Chapter 11) ! of species i for each phase is given by,! v ˆ fi = φˆi y i P For vapor mixture ˆl = γ x f f For liquid solution! i i i i VLE criteria (to be shown/derived in chapter 11), fˆ l = fˆ v i


 0 kommentar(er)
0 kommentar(er)
